Melampyrum nemorosum
Taxonomy: Spermatophyta, Scrophulariales, Orobanchaceae, Melampyrum
Published: 2012-12-04
Pollen Description
Shape, Size and Aperture
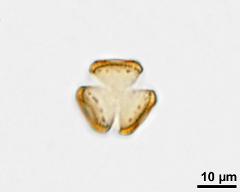
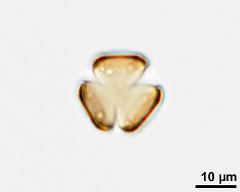
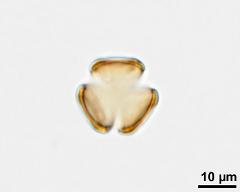
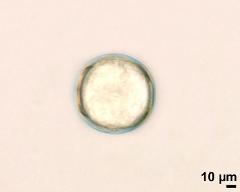
pollen unit: monad, dispersal unit and peculiarities: monad, size (pollen unit): small (10-25 µm), size of hydrated pollen (LM): -, shortest polar axis in equatorial view (LM): -, longest polar axis in equatorial view (LM): -, shortest diameter in equatorial or polar view (LM): -, longest diameter in equatorial or polar view (LM): -, pollen class: colpate, polarity: isopolar, P/E-ratio: -, shape: spheroidal, outline in polar view: circular, dominant orientation (LM): -, P/E-ratio (dry pollen): prolate, shape (dry pollen): -, outline in polar view (dry pollen): triangular, infoldings (dry pollen): interapertural area sunken, aperture number: 3, aperture type: -, aperture condition: colpate, aperture peculiarities: aperture membrane psilate
Ornamentation and Structure
LM ornamentation LM: no suitable term, nexine: present, sexine: present, SEM ornamentation SEM: no suitable term, suprasculpture SEM: -, TEM tectum: eutectate, infratectum: columellate, foot layer: discontinuous, endexine: compact-continuous, intine: bilayered, wall peculiarities: -, supratectal element: -
Miscellaneous
pollen coatings: pollenkitt, reserves in cytoplasm: lipids, cell number: 2-celled, Ubisch bodies: present
Annotations: Pollen grains 2- and 3-celled. Nuclei of anther-cells often contain calcium oxalate crystals.
Author(s) of diagnosis: Ulrich, S.
Pictures
Picture legend
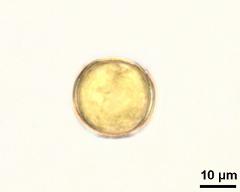
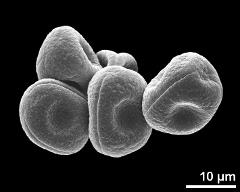
- habitus, photographer: Ulrich, S.
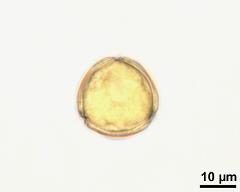
- flower(s), photographer: Ulrich, S.
- flower(s), photographer: Halbritter, H.
- no starch detected, equatorial view - dry, rehydrated (water), iodine, photographer: Ulrich, S.
- no starch detected, polar view - dry, Karnovsky-fixation (microwave), iodine, photographer: Ulrich, S.
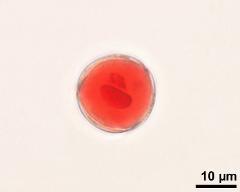
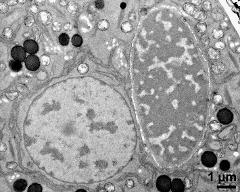
- two-celled pollen grain - dry, rehydrated (water), aceto-carmine, photographer: Ulrich, S.
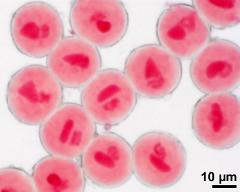
- two-celled pollen grains - dry, rehydrated (water) & glycerine, aceto-carmine, photographer: Ulrich, S.
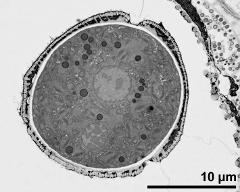
- acetolysed pollen in polar view, upper focus - dry, acetolyzed, unstained, photographer: Ulrich, S.
- acetolysed pollen in polar view, optical section - dry, acetolyzed, unstained, photographer: Ulrich, S.
- acetolysed pollen in polar view, lower focus - dry, acetolyzed, unstained, photographer: Ulrich, S.
- hydrated pollen, polar view - dry, rehydrated (water), unstained, photographer: Ulrich, S.
- hydrated pollen, equatorial view - dry, rehydrated (water), unstained, photographer: Ulrich, S.
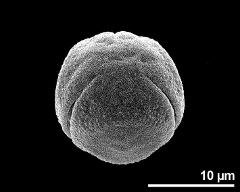
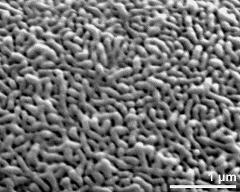
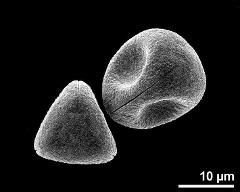
- polar view - dry, rehydration (water) & critical point dried & sputter coated with gold, photographer: Ulrich, S.
- polar view - fresh, rehydrated (water) & critical point dried & sputter coated with gold, photographer: Halbritter, H.
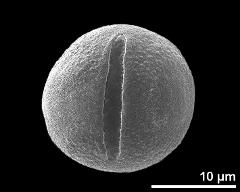
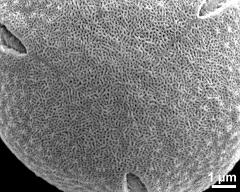
- equatorial view - fresh, rehydration (water) & critical point dried & sputter coated with gold, photographer: Ulrich, S.
- equatorial view - fresh, rehydrated (water) & critical point dried & sputter coated with gold, photographer: Halbritter, H.
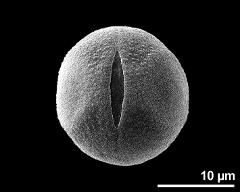
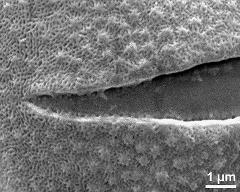
- aperture - fresh, rehydrated (water) & critical point dried & sputter coated with gold, photographer: Halbritter, H.
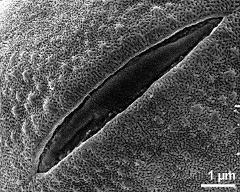
- detail of aperture - dry, rehydration (water) & critical point dried & sputter coated with gold, photographer: Ulrich, S.
- detail of aperture - dry, rehydration (water) & critical point dried & sputter coated with gold, photographer: Ulrich, S.
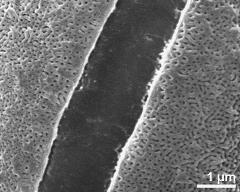
- exine surface, transition zone between aperture and interapertural area - dry, rehydration (water) & critical point dried & sputter coated with gold, photographer: Ulrich, S.
- detail of exine surface, wall thickening in interapertural area - dry, rehydration (water) & critical point dried & sputter coated with gold, photographer: Ulrich, S.
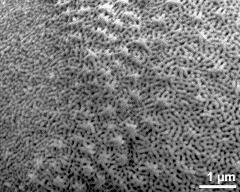
- detail of exine surface - dry, rehydration (water) & critical point dried & sputter coated with gold, photographer: Ulrich, S.
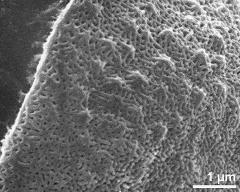
- polar area - dry, rehydration (water) & critical point dried & sputter coated with gold, photographer: Ulrich, S.
- polar area - fresh, DMP & critical point dried & sputter coated with gold, photographer: Halbritter, H.
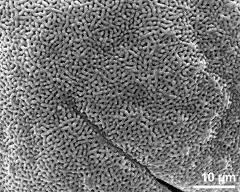
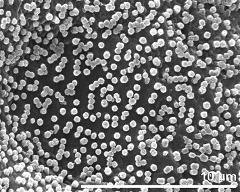
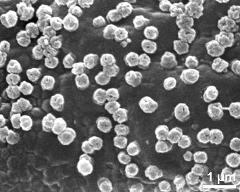
- dry pollen grains - dry, sputter coated with gold, photographer: Ulrich, S.
- dry pollen grains - dry, sputter coated with gold, photographer: Halbritter, H.
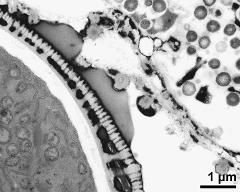
- Ubisch bodies on inner anther wall - dry, sputter coated with gold, photographer: Ulrich, S.
- Ubisch bodies on inner anther wall - dry, sputter coated with gold, photographer: Ulrich, S.
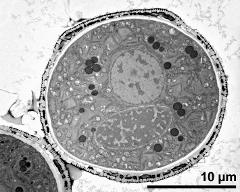
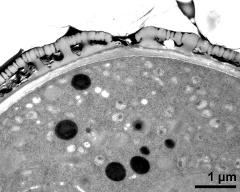
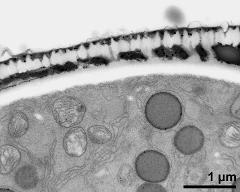
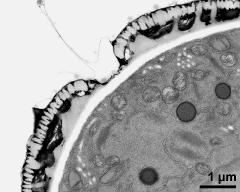
- cross section of pollen grain - fresh, glutaraldehyde & osmium & potassium ferrocyanide, modified Thiéry-test, photographer: Ulrich, S.
- cross section of pollen grain, and Ubisch bodies on loculus wall - fresh, glutaraldehyde & osmium & potassium ferrocyanide, modified Thiéry-test, photographer: Ulrich, S.
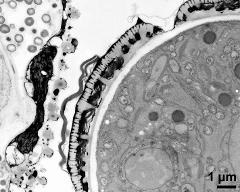
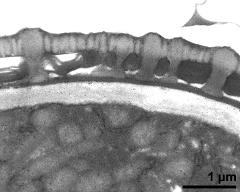
- pollen wall with pollenkitt and Ubisch bodies on inner anther wall - fresh, glutaraldehyde & osmium & potassium ferrocyanide, modified Thiéry-test, photographer: Ulrich, S.
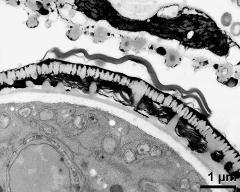
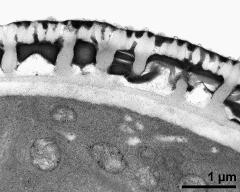
- pollen wall, aperture in cross section - fresh, glutaraldehyde & osmium & potassium ferrocyanide, uranyl acetate & lead citrate, photographer: Ulrich, S.
- pollen wall, interapertural area, pollenkitt and Ubisch bodies on inner anther wall - fresh, glutaraldehyde & osmium & potassium ferrocyanide, modified Thiéry-test, photographer: Ulrich, S.
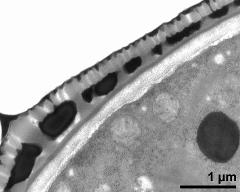
- pollen wall, interapertural area - fresh, glutaraldehyde & osmium & potassium ferrocyanide, lipid-test, photographer: Ulrich, S.
- pollen wall, interapertural area - fresh, glutaraldehyde & osmium & potassium ferrocyanide, lipid-test, photographer: Ulrich, S.
- pollen wall, interapertural area - fresh, glutaraldehyde & osmium & potassium ferrocyanide, uranyl acetate & lead citrate, photographer: Ulrich, S.
- pollen wall, transition zone between aperture and interapertural area - fresh, glutaraldehyde & osmium & potassium ferrocyanide, uranyl acetate & lead citrate, photographer: Ulrich, S.
- aperture in cross section - fresh, glutaraldehyde & osmium & potassium ferrocyanide, modified Thiéry-test, photographer: Ulrich, S.
- pollen wall, interapertural area, endexine clearly visible - fresh, glutaraldehyde & osmium & potassium ferrocyanide, potassium permanganate, photographer: Ulrich, S.
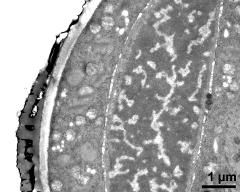
- vegetative nucleus and generative cell - fresh, glutaraldehyde & osmium & potassium ferrocyanide, modified Thiéry-test, photographer: Ulrich, S.
- pollen wall, interapertural area; pollenkitt and Ubisch bodies - fresh, glutaraldehyde & osmium & potassium ferrocyanide, modified Thiéry-test, photographer: Ulrich, S.
- Ubisch bodies on loculus wall - fresh, glutaraldehyde & osmium & potassium ferrocyanide, uranyl acetate & lead citrate, photographer: Ulrich, S.
Literature
- (1991) Materials on palinomorphological study of Pedicularis L. and Melampyrium L. genera. Biol J Armenia 44: 7-13
- (1984) A practical pollen guide to the British flora. Quaternary Res Bull Technical Guide I: 1-139
- (1986) Claves para la determinación de los polenes de las principales especies melíferas de la Península Ibérica. Orsis 2: 27-54
- (1942) Lehrbuch der Pollenanalyse. Handbücher der praktischen Vorgeschichtsforschung. Verlag Ferdinand Enke, Stuttgart 3: 195 pp
- (1877) Pollen. Hardwicke & Bogue, London : 92 pp, 24 pl
- (1961) An introduction to a scandinavian pollen flora. Grana Palynologica 2: 3-92
- (1963) An introduction to a scandinavian pollen flora - II. Almquist & Wiksell, Uppsala : 1-89
- (1964) Text book of pollen analysis. Scandinavian University Books, Munksgaard : 1-237
- (1890) Beiträge zur vergleichenden Morphologie der Pollenkörner. Breslau (Thesis) : 1-72
- (1897) Beiträge zur Biologie und Morphologie des Pollens. Sitzber Böhm Ges Wiss Prag Jahrb XXIII: 1-76
- (1956) Pollen grains of Japan. Hirokawa Publishing Co, Tokyo I-XII: 1-304
- (1982) Pollen grains in some Turkish Rhinantheae (Scrophulariaceae). Grana 21: 83-96
- (1977) The pollen morphology of plants in Ankara region IV. Scrophulariaceae. Commun Fac Sci Univ Ankara Ser C2 Bot 21: 131-143
- (1952) Atlas zur Bestimmung rezenter und fossiler Pollen und Sporen. Feddes Repert 133: 1-60, 57 pl
- (1978) Pollen dicotyledonearum Florae Partis Europaeae USSR. Lamiaceae - Zygophyllaceae. Nauka, Akad Sci USSR, VL Komarov Inst Bot : 184 pp
- (1971) Pollens et pollinisation de quelques Scrophularicacées Méditerranéennes. DEA, USTL, Montpellier : 59 pp
- (1927) Atlas und Bestimmungsschlüssel zur Pollenalytik. Bot Arch 19: 380-499, 50 pl
- (1989) Pollen morphology of the Orobanchaceae and rhinanthoid Scrophulariaceae. Grana 28: 1-18
- (1978) An illustrated guide to pollen analysis. Hodder & Stoughton Ltd, Kent : 133 pp
- (1991) Pollen analysis. Blackwell Scientific Publications. Second Edition : 216 pp
- (1994) Keys for the pollen of Ashiu, Central Japan. Contrib Biol Lab Kyoto Univ 28: 261-355
- (1992) Pollen et spores d'Europe et d'Afrique du Nord. Laboratoire de Botanique historique et Palynologie, Marseille : 520 pp, 446 pl
- (1939) Die Pollenkörner der in Deutschland wild wachsenden Scrophulariaceen. Ber Deutsch Bot Ges 57: 108-121
- (1973) Palynomorphs of Japanese plants. Spec Publ Osaka Mus Nat Hist 5: 1-60
- (1966) Illustrierte Flora von Mitteleuropa. Gustav Hegi 6: 2
- (1995) Pollen flora of China. Institute of Botany, Academia Sinica, Beijing. Second edition. : 461 pp, 205 pl
Copyright and Citation
Cite this publication as:
Ulrich S., Halbritter H. 2012. Melampyrum nemorosum. In: PalDat - A palynological database. https://www.paldat.org/pub/Melampyrum_nemorosum/206550;jsessionid=CC5709618E509CEC37F48FBC0BCE3063; accessed 2024-04-27